INTRODUCTION
Stroke is the second leading cause of death worldwide with an annual mortality rate of 6.55 million, which accounts for 11.6% of the total number of annual deaths. About 50% of stroke survivors suffer from chronic disability. The population-based Global Burden of Disease (GBD) study, conducted in 204 countries and territories from 1990 to 2019, showed that stroke was the third- leading cause of death and disability combined (5.7% of total disability-adjusted life years – DALYs). The number of DALYs due to stroke was estimated at roughly 143 million in 2019. Over the past three decades, the total number of stroke-related DALYs increased substantially, with a relatively small decrease in the high-income countries and large increases in the low- to upper-middle income ones [1].
There are two types of stroke, hemorrhagic stroke (HS) and ischemic stroke (IS). IS is due to the occlusion or blocking of blood vessels in the brain, and in 2019 it accounted for 62.4% of all strokes [1]. Early diagnosis is crucial; according to American Heart Association (AHA)/American Stroke Association (ASA) guidelines issued in 2019 for the early management of patients with acute ischemic stroke (AIS), non-contrast head computed tomography (NCCT, CT) or magnetic resonance imaging (MRI) is recommended as a part of the initial evaluation to exclude intracerebral hemorrhage (ICH) and identify patients who may benefit from the administration of recombinant tissue plasminogen activator (rtPA) or mechanical thrombectomy (MT). The intervention should be issued in the fastest achievable onset-to-treatment time. Preferably the door-to-needle (DTN) time for a patient treated with rtPA should be less than 60 minutes, whereas onset-to-needle (OTN) time should fall between 3 and 4.5 hours. The recommended door-to-puncture (DTP) time for patients treated with MT should be, respectively, less than 120 minutes and between 6-24 hours for onset-to-procedure (OTP) time [2]. Similar recommendations were formulated by the European Stroke Organization [3].
Human rtPA is a recommended treatment for patients with AIS. However, many stroke sufferers seek care too late to achieve the full benefit because the effectiveness of rtPA activator in the management of AIS diminishes significantly with time. The percentage of AIS patients receiving rtPA within the recommended time window (DTN time) in the United States is growing but still amounts to 50-60% [2]. Moreover, for patients with AIS due to large-vessel occlusion (LVO) the effectiveness of rtPA is only about 30%, and MT is recommended [4]. MT became the standard of care for patients with AIS with LVO, following the publication of the randomized clinical trial results in 2015 [5]. As with rtPA, studies showed that a faster administration of MT (measured by DTP time) is associated with better clinical outcomes. The reduction in DTN and DTP times are an important goal in the attempt to achieve effective treatment [2].
Lean management is an approach originating in the car manufacturing process derived from the Toyota Production System, which has recently been adapted by healthcare providers in Europe, North America, and Asia. The lean philosophy focuses on creating maximum value for patients by reducing waste and waiting times, and assessing the efficiency of resource use [6]. The concept of ‘lean’ concerns the streamlining of work processes while increasing quality and reducing waste. It focuses on the elimination of different types of waste, as originally defined by T. Ohno: waiting, transportation, inventory, motion, overproduction, excessive processing, and defects [7]. The process of creating value while eliminating waste is captured in the so-called six Lean Principles: Value – specify the value desired by the customer; Value stream – identify the value stream for each process and identify and eliminate all of the wasted steps; Flow – make the product flow continuously without rework; Pull – initiate process steps just-in-time; Perfection – identify imperfections and eliminate them through continuous improvement; Respect for People – create teamwork, eliminate the “blaming and shaming” work culture [8].
Lean management has developed many tools, such as Kanban, Total Preventive Maintenance (TPM), Setup Time Reduction, the Yamazumi Chart, Total Quality Management (TQM), 5S and Value Stream Mapping (VSM). VSM is a method used for the illustration of all process steps that allows the in-depth analysis and identification of value-adding and wasteful steps and results in process redesign. The three-phase VSM process includes preparing a current state map (CSM), future state map (FSM) and then an implementation plan [14]. CSM shows how the actual process operates, and requires walking down the production line. Then, FSM is prepared to show the root causes of waste and through process improvements. Finally, the implementation plan that lists the actions needed to achieve the goals of a project is developed [9].
Failure models, effects and criticality analysis (FMECA) is a proactive and systematic risk assessment method, which permits the evaluation of health system quality by identifying and preventing product and process failures before they occur [10]. FMECA also makes it possible to identify potential failure modes in systems, processes, products and services, and then prioritize them and decide on some actions to prevent or decrease the possibility of failures; finally, this process should be documented [11]. Both methods, VSM and FMECA, actively involve staff in analyzing and improving a process.
The objective of this study is to examine the relevance of using lean management tools in the management of AIS in hospital settings by conducting a systematic review of studies in this field. The aim of using lean management tools is to identify the causes of delays in DTN time or DTP time when dealing with AIS patients, in order to increase the percentage of patients being properly treated and, ultimately, improve healthcare outcomes.
METHODS
The MEDLINE and SCOPUS databases were searched, covering publications from 1st January 2000 to 20th September 2022. Studies assessing the implementation of lean management tools in mapping stroke treatment in inpatient settings that meet the inclusion criteria defined in Table 1 were sought.
Table 1
Inclusion and exclusion criteria
[i] DTN (door-to-needle) time–time from emergency department arrival to administration of rtPA, DTP (door- to- procedure) time–time from emergency department arrival to the initiation of mechanical thrombectomy, OTN (onset-to-needle) time–time from onset of symptoms to the administration of rtPA, OTP (onset-to-procedure) time – time from onset of symptoms to the initiation of mechanical thrombectomy, CT–non-contrast head computed tomography (NCCT), n.a.–not applicable
The search strategy used keywords related to lean management and value stream mapping synonyms (e.g. ‘lean thinking’, ‘lean concepts’, ‘lean approach’ etc.) combined with stroke synonyms (e.g. ‘acute ischemic stroke’, ‘cerebral stroke’ etc.) and was restricted to keywords that occur only in titles and abstracts.
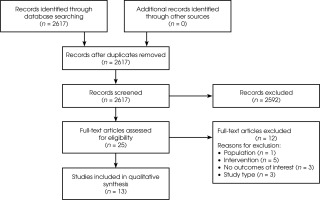
The search produced a total of 2617 hits. Independent analysts (of AZ, UC, MZ) assessed the titles and abstracts of the studies against inclusion criteria and the final decisions on inclusion or exclusion were made by four analysts. Finally, 13 studies were included. The PRISMA diagram of the process can be found in Figure I.
RESULTS
A total of 13 studies assessing the implementation of lean management tools in acute ischemic stroke treatment fulfilled the predefined inclusion criteria. The studies were mainly conducted in the United States [7]; others were for China [1], Singapore [1], England [1], Canada [1], Ireland [1] and Egypt [1]. Twelve of the studies have a prospective design; most of them were single-center [11] and the remainder were two-center [2]. All single-center studies and one two-center study used before-after methodology, where the results were reported separately for pre- intervention groups and post-intervention groups and then compared. Other two-center studies compared results between hospitals, namely academic and community hospitals [12]. Recombinant tissue plasminogen activator was used to treat AIS in 11 studies, and in two of these studies, rtPA was used in combination with mechanical thrombectomy. The two latest studies use only mechanical thrombectomy for AIS treatment. Value stream mapping was used in all 13 studies to systematically evaluate AIS treatment processes and identify areas where delays may occur. Outcome measures include mostly DTN time for rtPA treatment or DTP time for mechanical thrombectomy, though some studies reported on OTN time, door-to-imaging (CT, CBC or PTT) times, as well as patient outcomes in a modified Rankin Scale, length-of-stay, rates of symptomatic intracerebral hemorrhage (ICH), total hospital costs and inpatient deaths. A range of methods was implemented to improve patient-management workflow in hospital pathways, including a multitiered notification system that allows for communication between key members of an AIS team inside the hospital [4, 13], a system clock for electronic medical records (EMR), and 24-hour telestroke services in hospitals organized as a hub-and-spoke network that enables the consultation of AIS treatment between hospitals included in the network [14, 15]. The description of methods, sample sizes and observation periods for all studies included in the review are summarized in Table 2.
Table 2
Summary of methods, interventions and outcome measures used in studies included in the review
| Author, year of publication [Ref.], country | Methods | Intervention | Outcomes* measure |
|---|---|---|---|
| Hennebry, 2022 [13], Ireland | Prospective, before-after study Single-center Observation period: – Before phase: 2017 – After phase: two 9-month periods (18 months) Sample: n.a. | Treatment: MT and/or rtPA Lean interventions: VSM Other interventions: – An interdisciplinary process improvement team – Pre-alert system to ED staff | Door-to-CT-time (DTCT) DTN time Number of patients undergoing MT |
| El Nahas, 2021 [17], Egypt | Prospective, before-after study Single-center Observation period: 2016-2018 Sample: 1813 | Treatment: rtPA Lean interventions: VSM Other interventions: An interdisciplinary process improvement team | DTN time Percentage of patients receiving rtPA treatment mRS Score |
| Alcock, 2020 [18], Canada | Retrospective chart review study Single-center Observation period – Before phase: January 1, 2008 – December 31, 2010 – After phase: January 1, 2012 – December 31, 2014 Sample – Preintervention group: 102 patients – Postintervention group: 222 patients | Treatment: rtPA Lean interventions: VSM Other interventions: – Education and performance feedback were provided to neurologists, ED physicians, nurses, unit clerks, unit assistants, CT technologists, laboratory technologists – An interdisciplinary stroke committee established to address the workflow element of the quality improvement project – A pre-notification system with Emergency Medical Services notifying of ED of an incoming stroke patient | Primary outcomes: – DTN (median, % of pts. 60 min) – Door-to-CT time (DTCT) – CT-to-needle (CTTN) – Onset-to-door (OTD) – Onset-to-needle (OTN) – Inpatient mortality rates – Adverse events – Discharge location – Independence at discharge |
| Hill, 2020 [16], USA | Prospective, before-after study Single-center Observation period: – 11 months to achieve goal of ≤ 90 DTP time – Next 7 months to achieve goal of ≤ 80 DTP time Sample: – Preintervention group: 33 patients – Postintervention group: 71 patients | Treatment: MT Lean interventions: – VSM – Standardization – Change management Other interventions: – An interdisciplinary process improvement team including stroke coordinator, acute response nurse practitioners, ED nursing, neurointerventional radiology nursing and a technician – Neurointerventional radiology staff is available 24/7 through telemedicine, on-call system after hours and on weekends, with on-site nurse practitioners and physicians’ assistants – Communication tool ‘Vocera’ allows for an alert to be distributed quickly and simultaneously to all team members – Education materials including pictures of neurointerventional radiology and information on the location of key supplies | DTP time |
| Balcom, 2019 [21], USA | Prospective, before-after study Single-center Observation period: – Before phase: January-April 2017 – After phase: December 2017 Sample: n.a. | Treatment: rtPA Lean interventions: VSM Other interventions: – Interdisciplinary process improvement team including representatives from emergency room, laboratory, imaging services, pharmacy, endovascular procedure laboratory, critical care services, quality, and Emergency Medical Services (EMS) partners – Introducing pre-alert notification form EMS before patient arrives in the emergency room, pre-alteplase care performed by EMS team (i.e., intravenous access), dedicated stroke team wearing special purple blazers not to be disturbed by other staff members in emergency department | DTN time |
| Mong, 2019 [15], Singapore | Prospective, before-after study Single-center Observation period: – Before phase: April 9, 2012 – February 28, 2013 – After phase: March 1, 2013 – December 1, 2013 Sample: – Preintervention group: 33 patients – Postintervention group: 71 patients | Treatment: rtPA Lean interventions: – VSM of telestroke workflow – PDSA Other interventions: – 24-hour telestroke service implemented to facilitate the assessment of stroke patients by a neurologist from a remote location. Hub-and-spoke model using real-time audio-visual link allows for transfer of CT images – Formation of a multidisciplinary workgroup comprising clinicians, stroke case managers and radiology staff – Phase-by-phase review of the existing workflow was done to identify the reasons for delay | Primary outcomes: DTN time Secondary outcomes: – NIHSS – Rates of symptomatic ICH – Inpatient mortality rates |
| Goldstein, 2018 [4], USA | Prospective, before-after study Single-center Observation period: – Before phase: April 10, 2015 – April 11, 2016 – After phase: April 12, 2016 – May 10, 2017 Sample: – Preintervention group: 34 patients – Postintervention group: 28 patients | Treatment: MT Lean interventions: VSM Other interventions: – Implementation of a multitiered notification system: 3-tiered paging platform coordinated through the telephone operators – Lean process mapping was used to assess inefficiencies with multidisciplinary triage | Reduce in door-to-reperfusion (DTR) time mRS Score LoS on NCS Total hospital costs Inpatient deaths |
| Kansagra, 2018 [14], USA | Prospective, single-center (part of multi-hospital network, hub-and-spoke) Observation period: January 2015 – December 2015 Sample: 78 patients | Treatment: MT and/or rtPA Lean Interventions: VSM Other interventions: – Implementation of streamlined workflow to minimize DTP time – New, streamlined protocol for pre-thrombectomy management and workflow | Reduction in DTP time Reduction in onset-to-procedure time (OTP) Thrombectomy rates |
| Lawrence. 2018 [23], USA | Prospective, before-after study Single-center Observation period: – Before phase: July 2009 –June 2012 – After phase: July 2012 –December 2013 Sample: – Preintervention group: 78 patients – Postintervention group: 60 patients | Treatment: rtPA Lean Interventions: – VSM – Standardization – Visualization Other interventions: – Implementation of stroke model using a dedicated nurse with specialized stroke training; stroke specialist nurse became a coordinator of the multidisciplinary team – Implementation of the nursing flow sheet to keep track of and document critical benchmarks – Collect data for evaluate the effectiveness of intervention | DTN time (median, % of pts. 60 min) |
| Liang, 2016 [19], China | Prospective, before-after study Single-center Observation period: – Before phase: April 1st to August 31st, 2014 – After phase: Lean I: September 1st, 2014 to January 31st, 2015 Lean II: February 1st, 2014 to June 30th, 2015 Sample: – Preintervention group: 13 patients – Postintervention group: 43 patients (lean I: 23; lean II: 20) | Treatment: rtPA Lean interventions: – VSM – Visualization Other interventions: – A multidisciplinary team (Stroke Team) was established – Green pathway for stroke was organized by integrating treatment pathways in emergency department, neurology department, neurosurgery department, pharmacy department, laboratory department and radiology department | DTN time Percentage of patients with DTN ≤ 60 min |
| Prabhakaran, 2015 [12], USA | Prospective Two-center study (academic hospital vs. community hospital) Observation period: – Academic medical center (AMC): December 20, 2013 and January 10, 2014 – Community hospital: November 13, 2014 and December 11, 2014 Sample: n.a. | Treatment: rtPA Lean interventions: – VSM – Risk analysis using FMECA (failure modes, effects, and criticality analysis) Other interventions: A multidisciplinary team was established | ED triage delay Obtaining written consent from patient/ caregiver delay ED neurology examination delay CT delay Basing on these data risk priority numbers (RPNs) & criticality numbers (CNs) were calculated for the impact of the failure on DTN delay |
| Gill, 2014 [22], England | Prospective Single-center study Observation period: January 2012 – August 2014 Sample: – Year 2012: 115 – Year 2013: 138 – Year 2014: 100 | Treatment: rtPA Lean interventions: – VSM – PDSA Other interventions: – Ambulance pre-alert (telemedicine) to warn hospital that a patient that potentially requiring thrombolysis is transported – Re-write the existing pathways and clarifying the process – Personnel education and training – PDSA | DTN time Onset to door time Door to CT scan time OTN time |
| Ford, 2012 [20], USA | Prospective, before-after study Two-center study Observation period: –Before phase: January 1, 2009 to February 28, 2011 –After phase: March 1, 2011, to March 1, 2012 Sample: –Preintervention group: 132 –Postintervention group: 87 | Treatment: rtPA Lean interventions: VSM Other interventions: A multidisciplinary team (Stroke Team) was established. Protocol development. | DTN time (median, % of pts. ≤ 60 min) Onset-to-needle time Door-to-CT time Door-to-CBC time Door-to-PTT time Rates of symptomatic ICH mRS (0-2) LoS Stroke mimic |
CBC – complete blood count time, CT – computed tomography, DTN – door-to-needle, DTP – door-to-puncture, ER – emergency room, ICH – intracerebral hemorrhage, LoS – length of stay, mRS – modified Rankin Scale, MT – mechanical trombectomy, NCS – Neurocritical Care Service, NIHSS – National Institutes of Health Stroke Scale, PTT – partial thromboplastin time, VSM – value stream mapping, n.a. – not available, rtPA – recombinant tissue plasminogen activator, PDSA – plan-do-study-act
DTP was examined in two single-center before-after studies using the VSM approach to identify and improve areas of inefficiency and delay. In one study VSM and quality improvement led to a statistically significant decrease in DTP, from 147 to 39 minutes [14]. In Hill’s 2020 study [16], DTP was reduced from an average of 110-120 minutes to less than or equal to 90 minutes in 11 months, and to less than or equal to 80 minutes in the following 7 months. DTP was analyzed in one single-center before-after study, where the implementation of VSM and efficiency improvement resulted in a statistically significant reduction in this parameter from 170 to 127 minutes [6]. DTN time was assessed in nine studies, and statistically significant results were achieved in five. DTN time decreased from 68 to 40 minutes in El Nahas 2021 [17], 70.5 to 49 minutes in Alcock 2020 [18], 96 to 78 minutes in Mong 2019 [15], 90 to 47 minutes in Liang 2016 [19], and from 132 to 87 minutes in Ford 2012 [20]. In two studies the statistical significance was not reported [21, 22]. However, the baseline DTN time varied significantly between these studies, from 74.5 minutes [23] to 151 minutes, which shows that for some hospitals the room for improvement is much wider than it is in others, which may impact the significance of the results here. The percentages of patients treated with rtPA in the recommended 60 minutes or less from arrival at the hospital were assessed in three studies and statistically significant results were reported for all studies. The increase was from 28% to 52% in Lawrence 2018 [23], from 38.5% to 75% in Liang 2016 [19] and from 52% to 78% in Ford 2012 [20]. According to Gill 2014 [22] the DTN time was longer over the weekends and out of hours.
OTN time was measured in one study [14], although the difference in pre-to-post intervention was statistically insignificant. OTN time difference was assessed in two studies and reached statistical significance in both. A decrease in OTN time from 131 minutes to 111 minutes was reported in Ford 2012 [20].
Door-to-CT and CT-to-needle times were assessed in three studies, with statistically significant results reported in two of them [18, 20]. In one study the statistical significance was not reported [15]. Moreover, statistically significant results were reported in Mong 2019 [15] of an improvement in a National Institutes of Health Stroke Scale score at 24 hours and for rates of intracranial hemorrhages, as well as length-of-stay in a neurocritical care unit and total hospital costs in Goldstein 2018 [4]. Inpatient deaths were examined in four studies, though none of them reported statistical significance. All the results are summarized in Table 3.
Table 3
Summary of the studies’ results on most important outcome measures
| Endpoint | Study | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Preintervention group | Postintervention group | p-value | ||||||
| Time | n | Time | n | |||||
| Door-to puncture time (DTP) (min) | Hill 2020 [16] | 110-120 | n.a. | Phase I: ≤ 90 Phase II: ≤ 80 | n.a | n.a. | ||
| Kansagra 2018 [14] | 147 | 78 | 39 | 78 | < 0.001 | |||
| Door-to reperfusion time (DTR) (min) | Goldstein 2018 [4] | 170 | 34 | 127 | 28 | 0.02 | ||
| Door-to-needle (DTN) time (min) | Alcock 2021 [18] Median | 70.50 | 102 | 49 | 222 | < 0.001 | ||
| Hennebry 2022 [13] | 105 | na | Phase I (9-month): 35 Phase II (9-month): 50 | na | na | |||
| El Nahas 2021 [17] | 68 | na | 2017: 60 2018: 40 | na | ≤ 0.001 | |||
| Balcom 2019 [21] | 76 | na | 40.5 | na | na | |||
| Mong 2019 [15] | 96 | 33 | 78 | 71 | 0.003 | |||
| Lawrence 2018 [23] | Mean: 82 Median: 7.5 | 78 | Mean: 78 Median: 59.5 | 60 | 0.583, ns | |||
| Liang 2016 [19] | 90 | Lean I: 13 Lean II: 13 | Lean I: 55 Lean II: 47 | Lean I: 23 Lean II: 20 | Pre-lean vs. Lean I: 0.209 Pre-lean vs. Lean II: < 0.001 Lean I vs. Lean II: 0.046 | |||
| Gill 2014 [22] | 74.7 min | na | 2013 yr: 65.5 min 2014 yr: 49.0 min | na | na | |||
| Ford 2012 [20] | 60 (46-73) | 132 | 39 (28-56) | 87 | < 0.0001 | |||
| DTN ≤ 60 min (%) | Lawrence 2018 [23] | 28% | 78 | 52% | 60 | 0.05 | ||
| Liang 2016 [19] | 38.46% | 13 | Lean I: 60.67% Lean II: 75.00% | Lean I: 23 Lean II: 20 | Pre-lean vs. Lean I: 0.235 Pre-lean vs. Lean II: 0.015 Lean I vs. Lean II: 0.613 | |||
| Ford 2012 [20] | 52% | 132 | 78% | 87 | < 0.0001 | |||
| Onset-to-needle (OTN) time [min] | Kansagra 2018 [14] | 290 min | 78 | 212 min | 78 | ns | ||
| Liang 2016 [19] | 163 | 13 | Lean I: 192 Lean II: 170 | Lean I: 23 Lean II: 20 | Pre-lean vs. Lean I: 0.625 Pre-lean vs. Lean II: 0.011 Lean I vs. Lean II: 0.003 | |||
| Ford 2012 [20] | 131 (105-165) | 132 | 111 (80-158) | 87 | 0.016 | |||
| Door-to-CT time (min) | Alcock 2021 [18] | 23 | 102 | 18 | 222 | < 0.001 | ||
| Hennebry 2022 [13] | 38 | na | Phase I (9-month): 17 Phase II (9-month): 24 | na | na | |||
| Ford 2012 [20] | 16 (10-22) | 132 | 1 (0-4) | 87 | < 0.0001 | |||
| CT-to-needle (min) | Alcock 2021 [18] | 46.5 | 102 | 32 | 222 | < 0.001 | ||
| Improvement in NIHSS score at 24 hr (%) | Mong 2019 [15] | 25% | 32 | 47.9% | 67 | 0.031 | ||
| Modified Rankin Scale (mRS) Score 90-day | Value: 0 | El Nahas 2021 [17] | 31.8% | na | 35.2% | na | na | |
| Goldstein 2018 [4] | 0 (0%) | 34 | 5 (19%) | 28 | ns | |||
| Ford 2012 [20] | 49% | 132 | 43% | 87 | 0.34 | |||
| Value: 0-2 | El Nahas 2021 [17] | 49.9% | na | 73.2% | na | na | ||
| Liang 2016 [19] | 30.77% | 13 | Lean I: 73.91% Lean II: 75.00% | Lean I: 23 Lean II: 20 | Pre-lean vs. Lean I: 0.012 Pre-lean vs. Lean II: 0.012 Lean I vs. Lean II: 0.935 | |||
| Length of stay (LOS) on NCS (days) | Goldstein 2018 [4] | 6 | 34 | 3 | 28 | 0.006 | ||
| Total length of stay (LOS) (days) | Alcock 2021 [18] | 10 (5-22) | 102 | 5 (2-10) | 222 | 0.004 | ||
| Ford 2012 [20] | 4 (3-7) | 132 | 3 (2-6) | 87 | 0.056 | |||
| Total hospital costs ($) | Goldstein 2018 [4] | $161,458 | 34 | $100,083 | 28 | < 0.001 | ||
| Intracranial hemorrhages (%) | Alcock 2021 [18] | SICH<36 hours | 5 | 102 | 8.6 | 222 | 0.360 | |
| Life-treating | 0 | 102 | 1.8 | 222 | 0.314 | |||
| Mong 2019 [15] | 51.5 | 33 | 27.1 | 70 | 0.027 | |||
| Liang 2016 [16] | 0 | 13 | Lean I: 4.35 Lean II: 0 | Lean I: 23 Lean II: 20 | 0.482 | |||
| Ford 2012 [20] | 3.0 | 132 | 3.4 | 87 | 1.0 | |||
| Inpatient deaths (%) | Alcock 2021 [18] | 18.6 | 102 | 9 | 224 | 0.013 | ||
| Mong 2019 [15] | 6.1 | 33 | 7.0 | 71 | 1.000 | |||
| Goldstein 2018 [4] | 18.0 | 34 | 4.0 | 28 | 0.16 | |||
| Liang 2016 [19] | 7.69 | 13 | Lean I: 4.35 Lean II: 0.0 | Lean I: 23 Lean II: 20 | 0.491 | |||
Barriers to the provision of rapid rtPA treatment were identified as follows: inefficient patient flow, requiring them to be routed between particular departments (ED, trauma bay, CT scan room); inefficient use of available medical stuff (performing the same tasks simultaneously, distinct and parallel tasks); delays from patient arrival to the stroke team being informed; and delays in obtaining informed consent [12, 19, 20]. Despite these general issues, there are numerous causes of delays related to individual hospitals, so process redesign requires taking into account constraints and reality-based solutions [12].
Among the effective interventions identified, authors listed education and training for medical personnel, re-writing the existing protocols to clarify the process and make it more understandable, using electronic health records and telemedicine, including paramedics in stroke team activities (e.g. transfer to CT scan, electronic patient registration), strengthening the communication between ER staff and a stroke team, and standardizing routine conversations between patients and doctors. In one study, as a result of a VSM analysis pharmacists (to prepare tPA at bedside) and a social worker (to identify the witness and determine the onset of symptoms) were added to the multidisciplinary team, and point-of-care International Normalized Ratio (INR) testing was added to treatment pathway [20].
In Prabhakaran’s 2015 study [12], the DTN and DTP outcome measures were not addressed; instead, failure modes, effects, and criticality analysis (FMECA) were performed to compare the AIS treatment process between an academic medical center (AMC) and a community hospital (CH). At baseline, performance on the DTN ≤ 60 minutes was 75.7% in the AMC and 68.0% in the CH. A total of 42 AIS process steps were identified in the AMC, while 50 were taken in CH. Most process steps are related to the diagnosis phase. The significant delays found in care delivery at the AMC were due to incorrect diagnoses among walk-in patients, delayed CT scanning, and delays in the activation of the multidisciplinary stroke team. The most critical failures in the CH were delays in registration in the Emergency Department (ED) due to overcrowding, delays in obtaining consent for tPA treatment, and incorrect diagnosis of walk-in patients. Compared to AMC there were fewer staff involved in the AIS treatment process at the CH and fewer hierarchical barriers between nurses and physicians.
DISCUSSION
Lean management tools, mainly value stream mapping, have been applied in healthcare to improve the AIS treatment process. The benefit of using tissue plasminogen activator treatment or mechanical thrombectomy is that the therapeutic window is narrow and time in these situations is of the essence. Current guidelines indicate that rtPA should be administered in less than 60 minutes (DTN time) and that OTN time should be shorter than 4.5 hours. However, in practice these times usually exceed these recommended limits, resulting in the underutilization of effective medical treatment. In the studies analyzed here, the interventions applied resulted in a reduction of DTN time to less than 60 minutes in eight cases. Moreover, OTN time, analyzed in 3 studies, was within the time window of less than 4.5 hours. The results meet international standards and are consistent with current recommendations [2, 3, 24]. The findings suggest that identifying delays and implementing improvements can streamline the AIS treatment process. The systematic review conducted by Sharobeam et al. [25] concluded that pre-hospital factors are responsible for the largest delay in thrombolytic therapy. However, in-hospital factors are also important and can be divided into three groups: delays in assessment, delays related to imaging, and delays related to rtPA decision-making and administration. Delays in assessment are related to ED overcrowding, ED triage and long waiting times for registration for a CT scan and other laboratory tests. Patients transported by ambulance are less likely to experience delay than patients arriving in an ED themselves. The introduction of ambulance pre-notification to inform stroke team members helps reduce delays. A lack of availability of CT scanning, long waiting times for laboratory test results and obtaining informed consent can also generate significant delays. Sometimes younger patients are more likely to experience a delay because of atypical presentation.
According to the studies included in our systematic review, the most critical factors responsible for delays of AIS treatment with rtPA or mechanical thrombectomy are incorrect triage in the emergency department, delays in obtaining informed consent from patients, a lack of optimal workflow, delays in the decision-making process and laboratory tests, interdepartmental communication failures, timely preparation and staffing of neuro-interventional suites [4, 14, 15, 19, 26]. The studies presented here show that ischemic stroke patients with mild symptoms may be particularly at risk due to incorrect category assignments during triage in an ED. Efforts to reduce in-hospital delays should be focused on the optimization of patient workflow, including direct admission to the stroke unit and rapid imaging, and close collaboration between all participating healthcare professionals is required. Highly specialized personnel are of critical importance for reducing in-hospital delays [19]. Clearly defined roles allow for the parallel processing of tasks, resulting in the timely delivery of care [27]. In-hospital delays are caused by many factors, therefore a protocol- driven process that clearly defines the role of each stroke team member, using telemedicine or/and electronic health records, allows for the simultaneous rather than sequential delivery of care and may result in a significant improvement in the efficiency of care in time- sensitive stroke interventions. Another issue that can have a significant impact on the timing of the intervention is the time of day and the day of the week when the patient arrives in an ER. As some studies show, significant delays occur during the weekends and after the daily shift hours of the week, due to the limited number of specialist healthcare professionals present at those times. Regardless of the time of admission, statistically significant improvements in time parameters were observed, but in the evening/night and at weekends delays were significantly longer than in the daytime hours of weekdays. This is another important issue to consider when developing improvements in stroke management [27]. There is still room for improvement in lowering DTN or DTP time, even within high-performing centers already engaged in continuous quality improvement [12]. Studies show that a large volume of patients being treated with rtPA did not appear to adversely affect healthcare provider outcomes [20]. In fact, healthcare providers with high annual volume of activity achieved shorter DTN times than low-volume centers, so the organization of stroke centers may have the potential to significantly improve outcomes for AIS patients.
Telemedicine, as noted, can also be used to improve stroke management and shorten the duration of individual procedures. One of the studies showed that the use of telemedicine shortened the length of patient consultations when the neurologist was out of the institution by using teleconsultation [15]. A factor that can also have a significant impact on shortening time parameters is the development of rules for ambulance staff when notifying the patient’s condition to an ER. According to reports, the use of an alert containing information about the patient’s data, time of onset, vital signs, and approximate time of arrival at the hospital may be a solution to the time barrier resulting from the arrival of the stroke team after the patient’s admission to the ER. This information allows the medical staff to prepare ahead of time for the arrival of the stroke team and to receive the patient and as a result to implement procedures more quickly [22, 28].
Based on the analysis of the papers reviewed, we propose the adoption of the lean transformation framework, which may be anchored to improvements in the service procedure. Procedural change starts from the scoping and problem definition. The next step is the creation of a current state map. The current state map includes patients, critical resources, e.g. medical personnel (physicians, nurses, etc.), equipment, and information flow. An integrated part of the current state design process is waste identification, waste and problem quantification, and route cause analysis. At this step, all interfaces with other processes (inside and outside the hospital) should be identified. The next step is the preparation of a future state map. The implementation plan is prepared concurrently with the future state map. The foundation of lean transformation is workplace organization and process flow management. Lean change usually starts by implementing 5S, Visualization, and Standardization. After this, we can start eliminating waste from the process flow. The most critical and difficult aspect of all is changing people. Each change implementation needs time for the new process to stabilize. After the stabilization process, we can confirm whether or not the transformation has succeeded. Concurrently, during this stage, the organization should collect the lessons learned and best practices for further transformation.
Below we present a diagram showing the overall lean transformation process (Figure II).
Using the lean management approach in healthcare is quite different compared to the car manufacturing process, as treatment pathways are difficult to standardize because of various clinical factors that may affect the results. However, each hospital should create its process maps to identify non-value-added steps resulting from hospital-specific shortcomings and their unique characteristics.
The economic burden of ischemic stroke on healthcare systems is of increasing significance. An analysis carried out in 32 European countries showed that in 2017, 9 million people lived with a stroke, 1.5 million were diagnosed with stroke and 0.4 million died of stroke. This analysis estimated the total cost of stroke at €60 billion per year in 2017, of which €27 billion was spent on health systems, €5 billion was an annual expense for the care of stroke patients and €29 billion concerned the cost of informal care and loss of productivity due to premature death or absence from work [29]. Effective management can have a positive impact on improving treatment outcomes, resulting in reduced costs for the healthcare system. Both systemic thrombolysis and endovascular treatment appear to be major predictors of good clinical outcomes.
The studies reviewed here have several limitations; the sample size of most of them is small, which may not allow for the exposure of statistically significant changes and detection of potential differences in clinical outcomes. Most of the studies are single-center and were mostly conducted over a short period. However, most data for the post-lean implementations period were collected prospectively, and only for some historical control groups was the data collected retrospectively. Many of the causes of delays are related to individual hospitals, and the baseline times vary significantly between the studies, which makes the assessment of using lean management tools in reducing DTN and DTP time more difficult.
CONCLUSIONS
In light of the 2019 AHA/ASA recommendations, hospitals’ stroke systems should be developed to ensure the fastest achievable onset-to-treatment time for AIS patients. Establishing and monitoring target goals for DTN/DTP times can be beneficial in monitoring and enhancing system performance. Lean management can be a useful method for achieving key performance indicators in AIS in a way that is consistent with current guidelines. The results of this systematic literature review show the important relevance of value stream mapping in improving the process of AIS treatment by reducing in-hospital delays. Moreover, the research focused on implementing lean management tools, especially 5S, standards (checklists) in healthcare is growing, considering that more publications have appeared in recent years.