INTRODUCTION
Spinal cord ischemia or spinal cord infraction (SCI) is a relatively rare disease compared to cerebral stroke. Our knowledge as to its root causes, proper treatment for it and long-term prognosis is still inconclusive. SCI comprises 5.7% cases of acute myelopathy and 1-2% of all neurovascular events, although the exact incidence and prevalence remain unclear. Recent studies have shown that myelopathy related to ischemic diseases accounts for 14-18% of patients with transverse myelitis, suggesting the underdiagnosis of SCI [1]. It is diagnosed 100 times less often than cerebral stroke. SCI typically occurs between 50 and 70 years old on average [2]. It mainly presents as either anterior spinal artery syndrome or anterior spinal cord syndrome (ASCS) in up to 87.2% of cases [3-5]. Magnetic resonance imaging (MRI) with (diffusion-weighted contrast (DWI) should be considered for an initial diagnosis of spinal cord ischemia. A combination of DWI with ADC maps is recommended to distinguish SCI from other differential disorders. There are no established rules for the treatment of this condition.
Rapid intravenous administration of recombinant tissue plasminogen activator (rt-PA) might be an effective treatment for acute ischemic stroke, preventing permanent spinal dysfunction [6].
SPINAL CORD VASCULATURE – NEUROANATOMY
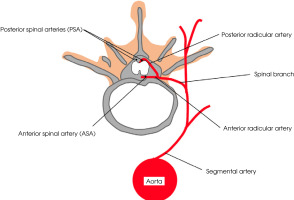
The principal blood supply to the spinal cord is via a single anterior spinal artery (ASA) and two posterior spinal arteries (PSA) (Figure I). The ASA provides blood to the anterior two-thirds of the spinal cord, and the PSA delivers blood to the posterior one-third of the spinal cord and posterior horn [7]. The second source of blood supply constitute radicular arteries, which originate from segmental arteries, and these in turn from ascending aorta. Segmental arteries give rise to radicular branches which penetrate the spinal cord bilaterally via intervertebral foramen. Each of the radicular arteries supply functionally separate parts of spinal arteries (Figure I). The first segment of the spinal cord (C1-Th3) is supplied mainly with branches that originate from the vertebral and carotid arteries. The thoracic region (Th3-Th7) is supplied with branches of posterior intercostal arteries and the superior intercostal artery. In the lumbosacral part (below Th8) the blood comes from artery of Adamkiewicz – the largest anterior radiculomedullary artery. The artery of Adamkiewicz (or arteria radiularis magna) typically arises from the left side of the aorta between T8 and L2 in 75% of people; it can, however, also be present above T8 [7-9].
THE MECHANISMS OF SPINAL CORD ISCHEMIA – PATHOPHYSIOLOGY
There exist two underlying major pathophysiological mechanisms for spinal cord infarction. Radicular artery territory infarcts are triggered by occlusion of the anterior or posterior artery, whereas central and transverse infarcts stem from systemic hypoperfusion. As for the main causes of SCI, aortic interventions and pathologies can be distinguished, accounting for around two-thirds of the cases [3]. Few reported cases of spinal cord ischemia are due to the existence of collateral circulation, which is created by vascular network. Ischemia is responsible for an inflammatory response and NMDA-mediated neuronal excitotoxicity [9, 10]. In various ischemic incidents, the presence of heat shock proteins might be observed [11, 12]. Spinal cord blood flow differs in particular areas of the cord [13]. Numerous pieces of research have revealed that, due to the density of motoneurons, and relative hypovascularity of the mid-thoracic cord, the thoracolumbar segment is most vulnerable to hypoperfusion. The cervical cord remains the second most affected region in up to 25% of patients [5].
ETIOLOGY
SCI is most probably closely associated with aortic diseases because of the vulnerability of the thoraco-lumbar spinal cord to hypoperfusion. A wide spectrum of other conditions may lead to spinal cord infraction: atherosclerosis, degenerative disease, systemic hypotension, cardiac embolism, coagulopathies, vasculitis, connective tissue disorders, and thrombophilia [4, 14-16]. In 20-30% the etiology remains unknown [3]. Ischemic myelopathy might result from mechanical compression exerted by an osteophyte on the anterior spinal artery, especially in the context of abrupt movement, physical exertion, or injury [17, 18]. Important risk factors for spinal cord ischemia are aortic surgeries, renal artery embolization, and aortic counterpulsation [19].
Aortic disorders as a primary cause of SCI
The most frequent and primary cause of SCI is aortic atherosclerosis, followed by other aortic conditions such as aortic dissection and complications of surgery performed on the aorta [3, 18]. Atherosclerotic plaques may lead to an impairment of the blood flow or constitute embolic material [19].
Aortic dissection (AD) when extends into the descending aorta resulting in insufficient perfusion of segmental arteries that supply the spinal cord [2]. Acute onset of paraplegia or paraparesis with a thoracic sensory level can be a dramatic presentation of dissection of the aorta. Dissection is usually preceded by the rapid onset of severe pain in the chest or back.
Midthoracic or lower are thoracic cord are most affected because they are supplied by the intercostal arteries that frequently suffer owing to aortic dissection [12]. A larger deficit may occur if the dissection involves the artery of Adamkiewicz, which supplies the levels of T10 to L2 in most patients [20]. Aortic aneurysm and aortic dissection are life-threatening conditions and should be investigated carefully in the presence of spinal cord infarction.
CLINICAL PRESENTATION OF SPINAL CORD ISCHEMIA
Symptoms of the disease depend on the level of spinal damage and its extent. Given the involvement of the vascular territory involvement, the clinical presentation of spinal cord infarction is with various degrees of dysfunctions [3].
Anterior spinal artery syndrome
The anterior spinal artery syndrome (ASAS) is the most frequent clinical presentation of SCIa [21, 22]. Symptoms typically include motor paralysis, and the loss of pain and temperature sensation below the level of the lesion. Proprioception, vibratory sense, and fine touch are preserved. Other symptoms include back pain, or autonomic dysfunction such as hypotension, neurogenic bowel or bladder, and sexual dysfunction. Initially, due to the spinal shock, paralysis is flaccid. Problems with controlling the vesical and rectal sphincters have also been reported [20, 23].
Posterior spinal artery syndrome
Posterior spinal artery syndrome (PSAS) occurs rarely and presents a diagnostic challenge. It is characterized by ipsilateral loss of proprioception, fine touch, pressure, and vibration below the lesion. The tendon and cutaneous reflexes are abolished. Pain and sensory sensation are preserved, excluding the part of cord segment which has suffered. In severe cases, large spinal cord lesions can also affect surrounding spinal tracts (the lateral part of corticospinal tract), resulting in bilateral movement deficit [19, 24].
Central syndrome of spinal cord injury
Central cord syndrome affects, in the vast majority of cases, the cervical spinal cord, mostly occurring after a fall with hyperextension of the patient’s neck [25]. Symptoms typically include paralysis or loss of fine control of movements in the arms and hands; however, movement of the legs with varying degrees of loss of pain, temperature, light touch, and pressure sensation below the level of injury and possibly urinary dysfunction remain intact.
Brown-Séquard syndrome
Hemodynamic disturbances and spondylosis might lead to the neurological condition called Brown-Séquard syndrome. The patient presents with weakness or paralysis and proprioceptive deficits on the side of the body ipsilateral to the lesion, accompanied by loss of pain and temperature sensation on the opposite side [26, 27].
DIAGNOSIS OF SPINAL CORD ISCHEMIA
Rapid diagnosis is essential if mortality or disability are to be prevented. Neurological examination is conducted initially, followed by neuroimaging [15]. MRI is a preferential method, although in up to 24% of patients the image might be entirely normal [10, 28]. Therefore, MRI should be repeated over consecutive days in order to rule out spinal cord compression. MRI imaging usually includes sagittal and axial T1 and T2-weighted sequences and diffusion-weighted imaging (DWI) [29]. DWI MRI is considered a highly sensitive method for detecting SCI within 8 hours of onset [12, 30]. A combination of DWI with ADC maps is recommended to distinguish SCI from myelitis and demyelinating disorders [6]. In the acute stage, ischemia presents as a restriction in the diffusion- weighted imaging of the spinal cord, a hyperintense signal on T2 and STIR, and an isointense one on T1 [31]. DWI-negative might be present in the hyperacute setting (less than 24 hours) and should not exclude the diagnosis, especially when other symptoms are present. “Owl eyes”, “pencil-like”, “positive anterior cauda” hyperintensity may support, but are not pathognomonic for, the diagnosis of SCI [28, 29]. Differential diagnosis includes a broad spectrum of myelopathies such as compressive, infectious, or inflammatory. Hyperintensities on T2-weighted images are not typical of ischemia and can also be revealed in various other conditions. In terms of multiple sclerosis, the lesions may occur in any part of the spinal cord but within the cervical enlargement plaques are most commonly found in the lateral columns. Additionally, in multiple sclerosis hyperintensities may also be revealed in T2-weighted MR images of the brain. In vascular congestion caused by spinal malformations, which is also difficult to distinguish from SCI, the ‘‘flow-void phenomenon” is frequently seen on T2-weighted images [32]. Other autoimmune disorders which may cause cord signal hyperintensities comprise systemic lupus erythematosus and Sjögren’s syndrome [14].
TREATMENT OF SPINAL CORD ISCHEMIA AND OUTCOMES
Spinal cord ischemia is a condition in which accurate treatment in necessary to achieve a favorable functional outcome. Unlike cerebral stroke, in which guidelines for management are well-established, the management of acute SCI is still under discussion. If the cause is ischemic etiology, the assessment of risk factors such as diabetes mellitus, hypertension, and hyperlipidemia should be carried out and accurate treatment should be implemented [19]. Treatment concepts have their origins in managing acute ischemic stroke and include airway and ventilation management, control of fever and glycemic, and anticoagulation, antiplatelet, and thromboprophylaxis therapy [33]. The administration of intravenous rt-PA within an adequate time window (to 4.5 hours from the onset of the symptoms) could be useful before a scheduled neurosurgical procedure. Only a few cases of using thrombolysis have been described in the literature [17, 34-37]. It would be advisable to set up a registry of patients with SCI that have been treated with rt-PA in order to evaluate the effectiveness of the treatment and establish management standards. If mechanical compression is present it requires immediate surgery. Thus, close cooperation between a neurologist and a neurosurgeon is essential. The effective preventive measure during aorta surgeries is the draining of cerebrospinal fluid (CSF), which improves spinal cord perfusion pressure. Hnath et al. [38] described of the 121 patients with thoracic stent graft placement. 56 patients (46%) underwent preoperative placement of a CSF drain, while 65 (54%) did not. No patient with drainage developed spinal ischemia (p < 0.05), compared with 5 episodes of SCI in 65 patients operated without drainage.
Importantly, an appropriate course of rehabilitation must be followed so as to prevent complications which may occur due to immobilization. These actions should ensure a return to mobility and independence in daily life activities [10].
CONCLUSIONS
Though it is an uncommon disease, SCI can be a severe, life-threatening condition. Aortic disease is the most common cause of SCI. Knowledge of spinal cord vasculature is required to fully understand the pathophysiology and symptomatology. In suspected SCI a rapid diagnosis, therapy and rehabilitation are crucial for long-term outcomes. Currently, recommendations derive from few described cases and guidelines regarding the management of cerebral stroke. The use of rt-PA could have an impact on the beneficial effect of treatment and functional outcome. However further evaluation and multi-center studies of ischemia of the spinal cord and its treatment would be advisable in neurological practice. In surgical cases, close cooperation between a neurologist and a neurosurgeon is necessary to provide prompt and combined appropriate management.