INTRODUCTION
There is currently an ongoing COVID-19 pandemic with an unpredictable time of its end. The global socio-economic problem caused by it is rapidly increasing, both worldwide [1-3] and in Ukraine. This has complicated the management of patients with chronic ophthalmic diseases due to the increase in the number of telemedicine consultations [2, 4], which will further have a negative impact on their compensation [1, 5, 6]. Then there will be “postCOVID” patients who will observe the consequences of this disease [7, 8] or the course of chronic general somatic pathology will change.
COVID-19 is an infectious disease that affects all organs and systems of the human body, having certain diagnostic features [9]. Due to the high contagiousness of COVID-19, at the beginning of the pandemic there were limitations in ophthalmological examination of patients, caused by a close doctor/patient contact during the examination with a slit lamp or ophthalmoscope [10], which affected the completeness of the eye lesion registration. Therefore, pathological conditions of the eye surface, which did not require a specific examination, were noted first.
In COVID-19 patients, conjunctiva changes were initially noted: hyperemia [11, 12], conjunctivitis [7, 11, 13-19], chemosis [19]; eye’s accessory organs changes: epiphora [12], blepharitis [7], dacryoadenitis [7], orbital cellulitis [7, 13], mucormycosis [7]. Over time, methods of protecting personnel from COVID-19 during a slit lamp examination [13, 18] made it possible to expand the COVID-19 ophthalmological manifestations, which included changes in the cornea – keratitis [18, 20], keratoconjunctivitis [13, 21] – and sclera: episcleritis [7, 13, 18]. During further studies, it was found that COVID-19 affected not only the anterior, but also the posterior part of the eye. The most common of these manifestations are: uveitis [1, 18, 22], vitreitis [18]; retina lesions: cotton wool spots [1, 11, 13, 18, 19, 23-24], intraretinal and petechial hemorrhages along retinal vessels [1, 11, 13, 19, 22-24], tortuosity and dilatation of retinal venules [1, 18, 24], CRVO [7, 18, 22], central retinal artery occlusion (CRAO) [7, 13], acute macular neuroretinopathy [7, 18, 22], COVID-19- induced maculopathy [18, 25]; and neuro-ophthalmological manifestations: bilateral acute optic neuritis [7, 26], papillophlebitis [7], Adie’s tonic pupil [7, 18], Miller Fisher syndrome and cranial nerve palsy [7, 13], neurogenic ptosis and a cerebrovascular accident with vision loss [7].
Some scientific publications divide COVID-19 retinal manifestations into early (acute phase of the disease: COVID-19 express test or COVID-19 PCR test – positive), during recovery (COVID-19 express test or COVID-19 PCR test – still positive), and “postCOVID” (COVID-19 express test or COVID-19 PCR test – negative). This distribution of COVID-19 retina lesions is relative, because it depends on the time of patients’ examination, chosen by the researcher. Therefore, some authors [7, 13, 18, 19, 22-24] refer cotton wool spots, petechial and intraretinal hemorrhages, CRVO, CRAO, COVID-19-induced maculopathy, tortuosity and dilatation of retinal veins to early/during recovery retinal manifestations. Authors [7, 24] include acute macular neuroretinopathy, tortuosity and dilatation of retinal veins in “postCOVID” retinal changes.
Despite the scale of this pandemic, there is a very limited amount of conflicting data on the relationship between COVID-19-associated retinal manifestations and features of the COVID-19 clinical course [16, 18]. Currently, the search for information that would make it possible to dia- gnose the COVID-19 course based on the condition of retinal vessels, as well as timely correcting ophthalmic manifestations or treatment of diseases that require long-term therapy and were diagnosed before COVID-19 [7], is relevant.
The aim of our work is to study the peculiarities of retinal changes’ structure and frequency according to fundoscopy data in patients with COVID-19 of different clinical course.
MATERIAL AND METHODS
The study was conducted on the basis of the Infectious Disease Department and the Regional Ophthalmology Center of the ME Volyn Regional Clinical Hospital of the Volyn Regional Council from October 2020 to December 2021. The study is prospective, uncontrolled and cross-sectional. In total, 117 patients (234 eyes) aged between 42 and 82 were under observation and hospitalized in the Infectious Disease Department of the clinic (positive COVID-19 PCR test). Patients with a retinal disease history were excluded from clinical observations. All clinical observations were carried out with the patients’ informed consent.
All patients underwent a general clinical examination: computed tomography of the lungs, measurement of blood saturation level, oxygen flow rate at which the normal level of blood saturation was maintained, blood pressure, heart rate and breathing rate, diagnosis of concomitant pathology (Table I). Vital signs and blood oxygen saturation levels were monitored from the moment of patients’ admission to the hospital until the moment of their discharge or death.
Table I
Patient groups’ clinical characteristics
[i] Note: at an oxygen flow rate of 0-5 l/min to maintain blood saturation at a normal level, oxygen was supplied through nasal cannulas, 5-10 l/min – through a mask without a respiratory bag, 10-20 l/min – through a mask with a respiratory bag, more than 20 l/min – both invasive and non-invasive ventilation.
All patients were divided into 4 groups according to the severity of the clinical course of COVID-19 (Table I). Group 1 (34 patients) included patients with an extremely severe course of COVID-19, with signs of severe pneumonia, acute respiratory distress syndrome, and treatment with artificial lung ventilation. Group 2 (26 patients) included patients with a severe course of COVID-19, with signs of severe pneumonia, plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90%; no artificial ventilation was used. Group 3 (30 patients) included patients with moderate severity of COVID-19, without signs of severe pneumonia, SpO2 ≥ 90%, with decompensated concomitant pathology. Group 4 (27 patients) included patients with moderate severity of COVID-19 without signs of severe pneumonia, SpO2 ≥ 90%, with compensated concomitant pathology [27, 28].
The fundus registration was carried out with the portable fundus camera Pictor Plus Fundus Camera and VistaView (Volk Optical). Photographic registration of the central part of the retina and retinal changes’ target registration were carried out. No instillation of drops was used when working with the non-mydriatic Pictor Plus Fundus Camera; the camera had a standard default light level for all iris types – according to the manufacturer’s instructions. During use of the VistaView mydriatic fundus camera, the patients were instilled with mydriatic drops – tropicamide 0.5% solution. Also for this camera different levels of illumination were used depending on the patient’s iris color: for dark brown eyes – a high level of illumination, for green and light brown – medium, for blue – low.
Statistical data processing algorithm
When conducting the analysis for quantitative indicators, their distribution was checked for normality (Shapiro-Wilk test). In the case of a normal distribution, the mean (–X) and standard deviation (± SD) were calculated, and for a non-normal distribution, the median (Me) and interquartile range (QI–QIII). For qualitative indicators, frequency (%) and, if necessary, a 95% probability interval (95% CI) were calculated. To compare the mean values in groups for quantitative indicators, in the case of a normal distribution, the analysis of variance was used, and in the case of a non-normal distribution, the Kruskal-Wallis test was used; the post hoc comparison was carried out according to Dunn’s test. The chi-square test was used to compare qualitative indicators, and the Bonferroni correction was used when comparing more than two groups. Calculations were performed for a two-sided critical region; the adopted level of significance was 0.05.
Two independent examiners were involved in indication of retinal changes. After that a classification table was created, from raw data in the spreadsheet, for two observers, and an inter-rater agreement statistic (κ) was calculated to evaluate the agreement between two classifications on nominal scales.
The κ value can be interpreted as follows (Altman, 1991):
| Value of κ | Strength of agreement |
| < 0.20 | Poor |
| 0.21-0.40 | Fair |
| 0.41-0.60 | Moderate |
| 0.61-0.80 | Good |
| 0.81-1.00 | Very good |
Our result of inter-rater agreement κ = 0.85 (95% CI: 0.77-0.94), which indicates a very good degree of consistency in the assessment of the indicator.
Bioethical standards
All materials were taken from the histories of patients of the Infectious Diseases Department of the ME Volyn Regional Clinical Hospital of the Volyn Regional Council and used in the article with the consent of the patients and/or their legal representatives. The study was approved by Ethics Committee of ME Volyn Regional Clinical Hospital of the Volyn Regional Council (protocol No. 2 on 15.09.2020).
RESULTS
It was discovered that out of 234 examined eyes, 47 (20.1%) had retinal changes, of which: 8 (17.0%) had narrowed retinal vessels, 14 (29.8%) had tortuosity and dilatation of retinal venules, 9 (19.1%) had cotton wool spots, 10 (21.3%) had intraretinal and petechial hemorrhages, 5 (10.6%) had CRVO, and 1 (2.1%) had prethrombosis of CRV. In addition to retinal changes, partial vitreous hemorrhage was detected in 2 patients (0.9%).
The identified retinal and ophthalmological changes were divided into those accompanied by a decrease in visual acuity in patients (clinically significant), 8 cases (17.0%): vitreous hemorrhage, prethrombosis of CRV, CRVO; and asymptomatic (clinically insignificant), 39 cases (83.0%): cotton wool spots, narrowed retinal vessels, intraretinal and petechial hemorrhages, tortuosity and dilatation of retinal venules.
Further, differences in retinal changes’ structure and frequency in patients with different clinical course of COVID-19 were investigated (Table II).
Table II
Retinal changes’ structure and frequency in patients with different clinical course of COVID-19
In group 1 (extremely severe patients), cotton wool spots (8.8%), narrowed retinal vessels (8.8%), petechial and intraretinal hemorrhages (23.5%), CRVO (Figure 1) (8.8%), tortuosity and dilatation of retinal venules (11.8%), vitreous hemorrhage (Figure 2) (5.9%), and prethrombosis of CRV (Figure 3) (2.9%) occurred quite often. Only in 29.4% of cases were no retinal changes observed.
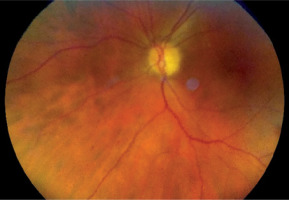
Figure 1
A) Patient V., 70 years old. COVID-19, extremely severe course, ARDS, RF III, lung parenchyma damage 72%, clinical observation group 1. Color fundus photo. CRVO. B) Patient N., 58 years old. COVID-19, severe course, RF III, lung parenchyma damage 53%, clinical observation group 2. Color fundus photo. CRVO
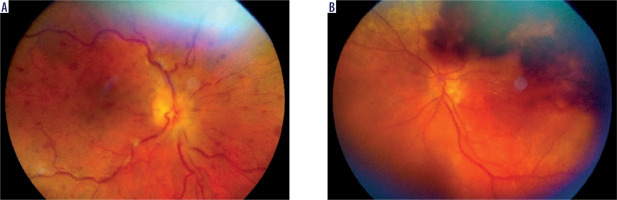
Figure 2
Patient L., 72 years old. COVID-19, extremely severe course, ARDS, RF III, lung parenchyma damage 68%, clinical observation group 1. Color fundus photo. Vitreous hemorrhage
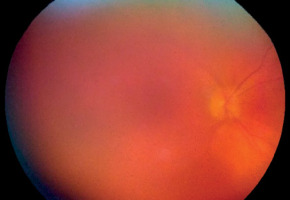
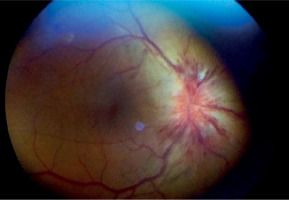
Figure 3
Patient R., 68 years old. COVID-19, extremely severe course, ARDS, RF III, lung parenchyma damage 67%, clinical observation group 1. Color fundus photo. Prethrombosis of CRV
In group 2 (severe patients), the frequency of retinal change distribution was similar to group 1: cotton wool spots (Figure 4) (11.5%), narrowed retinal vessels (7.7%), petechial and intraretinal hemorrhages (Figure 5) (7.7%), tortuosity and dilatation of retinal venules (7.7%), CRVO in 3.8% of cases. We did not observe any cases of prethrombosis of CRV or vitreous hemorrhage in patients of this group.
Figure 4
Patient A., 53 years old. COVID-19, moderate severity with decompensated concominant pathology, RF II, lung parenchyma damage 41%, clinical observation group 3. Color fundus photo. Cotton wool spots along the superior temporal vascular arcade
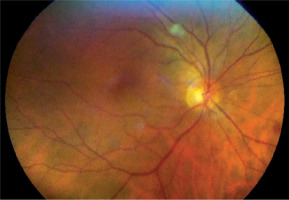
Figure 5
Patient S., 62 years old. COVID-19, severe course, RF III, lung parenchyma damage 48%, clinical observation group 2. Color fundus photo. Petechial hemorrhages on the retina middle periphery
In group 3 (patients with moderate severity and decompensated (a condition in which the concomitant pathology affects the course of the main disease and is uncorrected in all its indicators) concomitant pathology), cotton wool spots were diagnosed in 10% and CRVO in 3.3% of cases. The maximum number of patients without retinal changes was observed in this group – 86.7%. In patients with moderate severity of COVID-19 and compensated (a condition in which the concomitant pathology does not affect the course of the main disease and is corrected for in all its indicators) concomitant pathology (group 4) expansion and tortuosity of retinal venules was found in 29.6% (Figure 6), narrowing of retinal vessels in 11.1% (Figure 7), and no retinal changes in 59.3% of cases.
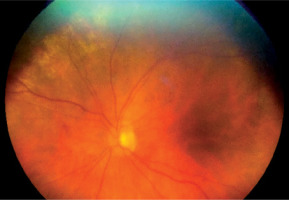
Figure 6
Patient T., 59 years old. COVID-19, moderate severity with compensated concominant pathology, lung parenchyma damage 28%, clinical observation group 4. Color fundus photo. Dilatated retinal venules
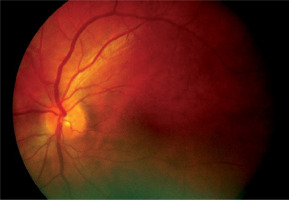
Figure 7
Patient O., 65 years old. COVID-19, moderate severity with compensated concominant pathology, lung parenchyma damage 38%, clinical observation group 4. Color fundus photo. Narrowed retinal vessels
During the analysis, a difference was revealed in the retinal change distribution of the frequency of detection for patients of the 4 groups (p<0.001 according to the chi-square test). At the same time, a statistically significant (taking into account the Bonferroni correction) difference in the distribution (p < 0.05) of retinal changes between group 1 and groups 3 and 4 was detected. No statistically significant difference in the distribution between group 1 and group 2 was found (p > 0.05).
The analysis revealed a difference in the distribution of the frequency of detection of clinically significant retinal changes with different COVID-19 severity (Table II). Figure 8 presents an interval estimate of frequency of detection of clinically significant retinal changes in the 4 groups of patients.
Figure 8
An interval estimate of clinically significant retinal changes frequency of detection in 4 groups of patients
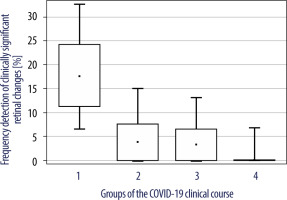
A significant trend of decreasing risk of clinically significant retinal changes with decreasing COVID-19 severity was observed (p = 0.007 according to the chi-square test for ordered gradations). There were no clinically significant retinal changes in patients of group 4. Group 2 and group 3 patients had a low incidence of clinically significant retinal changes, 3.8% and 3.3%, respectively, indicating a low probability of developing systemic COVID-19 extrapulmonary vascular complications. Group 1 patients had a very high incidence of clinically significant retinal changes – 17.6%.
DISCUSSION
The most controversial issue among ophthalmological manifestations of COVID-19 is the peculiarities of structure and distribution of retinal changes in different clinical courses of the disease. Today, the division of retinal manifestations into early and “postCOVID” is described. A number of authors [7, 13, 18, 19, 22-24] have considered cotton wool spots, petechial and intraretinal hemorrhages, CRVO, CRAO, COVID-19-induced maculopathy, tortuosity and dilatation of retinal venules as early and/or during recovery retinal manifestations. Acute macular neuroretinopathy, tortuosity and dilatation of retinal venules are included in the “postCOVID” manifestations [7, 24]. At the same time, there are insufficient data on correlations between changes of patients’ retinal vessels and different clinical course of COVID-19. Regarding the division of above-mentioned changes into clinically significant and clinically insignificant and their connection with the COVID-19 severity, the results are quite contradictory.
Thus, cotton wool spots and petechial hemorrhages along the vascular retinal arcades were found in COVID-19 patients, all were hospitalized but did not require resuscitation measures, severe COVID-19 was not noted, and the existing accompanying pathology was fully compensated [11, 19, 23]. In contrast, Mack, Fraser-Bell and Lima with co-authors stated that cotton wool spots, petechial hemorrhages along vascular retinal arcades and retinal venule dilatation are not pathognomonic signs of COVID-19, but can also appear in other infectious diseases as a component of the body’s response to the inflammatory process [1, 20]. These patient groups are debatable, as no information is provided on COVID-19 severity or the presence of ophthalmological complaints in patients. Sen et al. also described cotton wool spots and petechial hemorrhages along the vascular retinal arcades in patients with severe course of COVID-19, but did not determine the presence of ophthalmological complaints in patients (decreased visual acuity) [7]. Invernizzi et al. noted that cotton wool spots and petechial hemorrhages along the vascular retinal arcades, dilatation and tortuosity of retinal venules occured in patients with moderate severity of COVID-19, but like the previous authors they did not note the presence of ophthalmological complaints in patients (decreased visual acuity) [24].
CRVO is described [7, 18, 22] in patients with a severe course of COVID-19 and complaints of rapid decrease in visual acuity, which affects the clinical significance of this retinal manifestation. There are also cases of CRAO during severe COVID-19 and patients’ complaints of a rapid, painless decrease in visual acuity, which also renders such a manifestation clinically significant [7, 13]. Especially interesting is COVID-19-induced maculopathy in the case of a mild course of infection with complaints of a gradual decrease in visual acuity, which makes this extrapulmonary manifestation also clinically significant [18, 25].
All things considered, we conclude that the data on retinal changes in COVID-19 structure are quite contradictory. Patients’ groups take into account an incomplete list of clinical signs, according to which it can be clearly said that certain retinal manifestations can be present only in patients with a severe course, and others only in cases of mild or moderate severity. Also, the presence of ophthalmological complaints in patients is not always taken into account.
Statistical processing of our study data revealed certain correlations between the COVID-19 severity and the spectrum of retinal changes. Thus, we can state that, comparing patients with an extremely severe or severe course, the list of ophthalmological manifestations does not differ and contains both clinically significant (CRVO, prethrombosis of CRV, partial vitreous hemorrhage) and clinically insignificant changes (tortuosity and dilatation of retinal venules, narrowed retinal vessels, cotton wool spots, petechial and intraretinal hemorrhages). This information suggests that in the cohort of patients from these groups there are prerequisites for the occurrence of other COVID-19 extrapulmonary vascular complications. Mostly patients with COVID-19 of moderate severity and decompensated concomitant pathology had clinically insignificant retinal changes (cotton wool spots) and there was only one case of clinically significant changes (CRVO). In our opinion, it is possible to assume a reduction in the risk of other COVID-19 vascular complications. Patients with moderate COVID-19 and compensated concomitant pathology had only clinically insignificant retinal changes (narrowed retinal vessels, tortuosity and dilatation of retinal venules), which eliminates any risk of COVID-19 extrapulmonary vascular complications.
Such facts encourage further research into the structure of retinal changes, taking into account COVID-19 metabolic changes, their relationship with the severity of COVID-19 and the presence of certain retinal manifestations. This would make it possible to timely adjust COVID-19 treatment and avoid various “postCOVID” ophthalmic consequences in patients.
CONCLUSIONS
The structure and frequency of retinal changes in patients with COVID-19 depend on the severity of clinical course of the disease. Retinal changes (prethrombosis/thrombosis of CRV, cotton wool spots, narrowed retinal vessels, intraretinal and petechial hemorrhages, tortuosity and dilation of retinal venules) and vitreous hemorrhage occur significantly more often in patients with an extremely severe course of COVID-19 than in patients with a milder course (p < 0.05). As the severity of the disease decreases, the spectrum of diagnosed retinal changes becomes different. So, in patients with a moderate course of COVID-19 and compensated concomitant pathology, only narrowed retinal vessels, tortuosity and dilatation of retinal venules were present.
A significant trend of decreasing risk of clinically significant retinal changes with decreasing disease severity was observed (p = 0.007 according to the chi-square test for ordered gradations). Clinically significant retinal changes (prethrombosis of CRV, CRVO), as well as vitreous hemorrhage were most often detected in patients with extremely severe and severe courses of COVID-19 and were absent in patients with a course of moderate severity and compensated concomitant pathology.

 ENGLISH
ENGLISH




