Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal malignancies of the hematopoietic system, characterized by ineffective hematopoiesis with peripheral cytopenias. During the diagnosis, peripheral blood morphology, and cytological and histopathological examination of the bone marrow are necessary to assess the degree of quality disorders of cells of particular cell lines called dysplasia. Cytochemistry allows the detection of iron deposits around the nuclei of erythroblasts (sideroblasts) [1]. Genetic mutations have great importance in the pathogenesis of MDS, affecting various stages of the cell cycle and determining the progression of the disease. They also allow the classification of MDS subtypes Among others, MDS with ring sideroblasts are distinguished, the diagnosis of which requires the detection of the SF3B1 mutation, or MDS with isolated deletion of 5q, where cytogenetics are showing del(5q) alone or with one additional abnormality except monosomy 7 or del(7q) [2]. Due to the non-specific clinical symptoms, the diagnosis of MDS poses many difficulties, and dysplastic cells may be misdiagnosed during automatic leukocyte separation.
The aim of the study was the detection of differences between automatic leukocyte separation and microscopic evaluation of a blood smear in a patient with MDS.
Case description
A 68-year-old patient was admitted to the Department of Internal Diseases and Endocrinology of the University Clinical Centre of the Medical University of Warsaw. The patient had a history of pancytopenia in the course of myelodysplastic syndrome and vascular bleeding diathesis. During his stay in the department biochemical laboratory tests showed metabolic alkalosis, chronic kidney disease in the G2 state, acute phase state (elevated C-reactive protein 186 mg/l, procalcitonin 22.0 µg/ml and IL-6 was 360 pg/ml) and hyperparathyroidism (elevated parathyroid hormone 164 pg/ml, alkaline phosphatase 166 IU/L, reduced calcium 2.70 mmol/l and magnesium 0.54 mmol/l ions and phosphates 0.64 mmol/l). The chronic kidney disease and eGFR - CKD-EPI was 54 ml/min/1.73m^2.
The patient was diagnosed with myelodysplastic syndrome with isolated 5q deletion. The results of flow cytometry of bone marrow cells were the following: 44% monocytes/macrophages CD45(+) CD14(+) SSC(á); 34% granulocytes CD45(dim) SSC(á); 17% lymphocytes – heterogenous population of T lymphocytes CD5(+) CD19(-) including 71% CD4(+) and 31% CD8(+); 4% polyclonal B lymphocytes: CD19(+) CD5(-). It excluded monoclonal expansion of NHL B-cell lymphoma.
The complete differential WBC counts (5-DIFF) performed on automated hematology analyzer Sysmex XN-3000 with a DI-60 module (Kobe, Japan) revealed persistent pancytopenia: WBC 1.81/ 1.58/ 2.4 x106/L, NEUT 0.90/ 0.66/ 0.78 x106/L, HGB 7.4/ 7.2/ 5.7 g/dL, PLT 5/ 6/ 5 x106/L. The results of complete blood count analysis are shown in Table 1.
Table 1
Summary of cell count results and complete blood counts
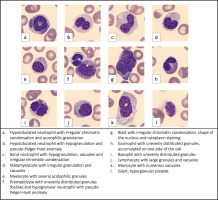
Despite the blood transfusions, levels of hemoglobin and platelets remained low as a result of intravascular hemolysis and profuse gastrointestinal bleeding. The blood smear showed features of dysplasia, such as nucleus/cytoplasm maturation asynchrony, irregular chromatin condensation in granulocytes, hypo- and hypersegmentation of granulocyte nuclei, hypogranulation and hypergranulation of granulocyte cytoplasm, and uneven distribution of granules in the cytoplasm of eosinophils. Hypolobulated neutrophils with azurophilic granulation and pseudo-Pelger-Huet anomaly were observed, as well as hypogranulated band neutophils with vacuoles. Irregular granulation was present in metamyelocytes, promyelocytes, eosinophils and basophils. Blasts and neutrophils had visible irregular chromatin condensation. Acidophilic granules were observed in myelocytes. Lymphocytes with large granules were also present. Monocytes had numerous vacuoles, whereas platelets were large and hypergranular (Figure 1).
The largest variances between the automatic separation of leukocytes and the microscopic smear concerned the percentage of eosinophils, neutrophils, and atypical lymphocytes. In addition, the microscopic smear revealed the presence of eosinophils at various stages of maturity, starting from the myelocyte stage.
Case analysis
Nowadays, the first line of hematological diagnostics is morphology with automatic leukocyte smear, which allows for the initial assessment of the white blood cell lineage. Analyzers using fluorescence flow cytometry are characterized by high accuracy and the ability to differentiate cells. However, in demanding cases – such as the one presented in this report – they will not replace manual microscopic smear and assessment by a laboratory diagnostician or other specialist. Bone marrow cytogenetic and molecular tests can also be performed as an auxiliary. Clinically, by using both methods when necessary, hematological patient would be brought closer to a clear diagnosis, which will enable appropriate treatment in subsequent steps. This case also shows how important and essential trained laboratory workers are in health care – they supervise the results given by analyzers and verify their correctness.
Shamila et al. [3] compared the results of automatic analysis using the Siemens ADVIA 120i analyzer using fluorescence flow cytometry and the manual method of samples from healthy patients. No significant differences between the methods were found, which confirms that the automatic smear may be sufficient in healthy patients, but not necessarily for pathologies [3].
Merino et al. [4] confronted results obtained with Mindray MC-80, a new automated analyzer used for digital imaging of white blood cells, and results received by light microscopy. Researchers used a total of 445 samples, which included normal samples (194) as well as ones from patients suffering from hematological disorders (251). They proved that Mindray MC-80 is efficient in distinguishing WBC both in samples with normal and pathological blood cells. The only differences applied to the range of basophils and IG [4].
The diagnostic criteria for myelodysplastic syndromes include a wide range of tests and methods, but changes in blood morphology and blood smear are still one of the first indicators of the disease. This group of diseases is characterized by dysplasia of blood lineages, which means that automatic methods will not always be able to adequately differentiate the cells presenting non-specific dysplastic changes [5].
Microscopic evaluation of a blood smear can currently be performed using automatic methods based on taking microscopic photos of counted cells and then qualifying them to specific cell groups [6]. Evaluation of cells under a light microscope allows for a more accurate characterization of the morphology of the cells being assessed, which remains the gold standard for leukocyte qualification [7].
The automatic analyzers used also have problems with assessing discrete morphological changes, e.g. cytoplasmic hypogranulation occurring in patients with myelodysplastic syndrome and signs of dysplasia in the granulocytic system, as evidenced by research conducted by Zini et al. [8]. Peripheral pancytopenia occurring due to quantitative disorders may also pose a problem in counting and assessing cells in the preparation, which is most often compensated by the assessment of two microscopic preparations [9].
Implementation of artificial intelligence-based instruments in hematology laboratories requires evidence of the effectiveness of pathological cell classification.
Studies by Hollenstein et al. [10] have demonstrated that assessment using the DI-60 automated analyzer shows agreement with microscopic assessment for patient samples without pathology. In the case of cells present in lower numbers in the blood, such as basophils, eosinophils and immature, atypical and blast cells, it requires verification employing manual methods using a light microscope. Features of the digital blood count analyzer (e.g. limited field of view) may also result in lower sensitivity for cells that are more likely to accumulate at the edge of the smear, such as monocytes or plasma cells [10].
The analysis of a microscopic blood smear poses many difficulties, and available hematological analyzers and the digital assessment used can be an element of support in the screening diagnosis of patients with suspected hematological growths.
Conclusions
Automatic blood smear analysis compared to manual analysis can show significantly different results. To diagnose diseases of the hematopoietic system, it is recommended to verify the results of the tests using the microscopic method. Bone marrow cytogenetic and molecular tests can also be performed as an auxiliary.