INRODUCTION
Endophthalmitis is an infectious condition caused by pathogenic microorganisms, affecting the internal structures of the eye, characterized by severe, progressive inflammation in the vitreous body and/or aqueous humor [1]. In most cases (92-98%), the inflammation is exogenous, resulting from a disruption of the ocular structural integrity due to trauma, surgery, or corneal ulcers. This creates portals of infection, allowing microorganisms to enter the inside of the eye.
A much less common type is endogenous endophthalmitis, accounting for 2-8% of cases [2-4]. It occurs as a result of microorganisms spreading through the bloodstream from distant sites of inflammation in the body, such as endocarditis, meningitis, urinary tract infections, or abscesses. Endo- genous inflammation is characterized by a more severe course and a poorer prognosis. It is often linked to delayed diagnosis, more virulent causative microorganisms, and severe overall condition of affected patients. The etiology of the infection is diverse. In developed countries, the most common causative organisms are Gram-positive bacteria and Candida fungi. In contrast, Gram-negative bacteria are responsible for the majority of cases in Asia [2, 5, 6]. Endophthalmitis caused by Klebsiella pneumoniae is associated with a very poor prognosis and an unfavorable disease course [7].
Early diagnosis and immediate initiation of aggressive therapy are essential for achieving a satisfying therapeutic outcome.
CASE REPORT 1
The patient, a 66-year-old man in a moderate general condition, was transferred from the Department of Internal Medicine of a nearby hospital due to sudden onset of binocular blindness lasting about a dozen hours and pain in the left eye. The patient had a hectic fever. No history of trauma, surgery, or ophthalmic disease was found; the patient was receiving chronic treatment for atrial fibrillation.
On admission, the visual acuity in the right eye (Vod) and left eye (Vos) was light perception with full projection. Physical examination revealed deep ocular irritation with corneal haze and an exudative membrane within the pupil of the right eye, and an exudate and pus accumulation in the anterior chamber of the left eye. B-scan ultrasound revealed multiple hyperechoic poorly mobile echoes in the vitreous as well as vitreoretinal tractions.
Based on the clinical presentation, laboratory tests, and diagnostic imaging, endophthalmitis of endogenous origin was suspected.
Laboratory tests revealed elevated inflammatory markers: C-reactive protein (CRP) 249.3 mg/l, procalcitonin 60.85 ng/ml, ESR 62 mm/h, leukocytosis 10.74 thousand/μl. Moreover, elevated liver enzyme levels were noted [aspartate aminotransaminase (AST) 45 U/l; alanine aminotransferase (ALT) 64 U/l], along with significantly increased D-dimer levels (8,796 ng/ml).
Empirical broad-spectrum intravenous antibiotic therapy (ceftazidime, amikacin, metronidazole, ciprofloxacin, and fluconazole) and topical treatment (moxifloxacin, loteprednol, dexamethasone, gentamicin, atropine) were initiated. On the second day of hospitalization, treatment with daily intravitreal injections of 1 mg/0.1 ml of vancomycin and 2.25 mg/0.1 ml of ceftazidime per each injection was also commenced. On the third day, Vod was determined as counting fingers at 20 cm, while Vos from the second day of hospitalization was evaluated as no light perception.
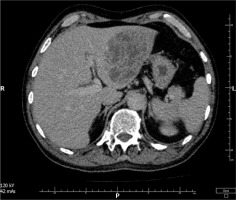
Computed tomography (CT) of the abdominal cavity revealed a large heterogeneous structure with numerous fluid-filled spaces in the left lobe of the liver, measuring 95 × 98 × 80 mm (Figure 1). Consequently, a liver abscess was identified as the precipitating cause of the septic condition.
On the fourth day of hospitalization, following transfer to the General Surgery Department, a laparotomy was conducted. During the procedure, purulent content was evacuated, and drainage of the liver abscess cavity was performed. Klebsiella pneumoniae bacterium was isolated from blood and surgical specimens.
Following the procedure, the patient experienced respiratory failure and was transferred for treatment to the Intensive Care Unit, where he received mechanical ventilation, blood products, parenteral nutrition, and continued intravenous antibiotic therapy (ceftazidime, doxycycline, erythromycin, fluconazole). Topical treatment and intravitreal injections were also continued. The patient’s overall condition stabilized 30 days after the procedure, with local stabilization achieved after 15 days.
On the day of discharge (i.e. day 33 of hospitalization), the right eye was calm and painless, with a clear anterior chamber and post-inflammatory floaters present in the vitreous humor. The left eye remained painless, irritated, with pus present in the anterior chamber. Visual acuity in the right eye was determined as counting fingers at 2 m, while the left eye remained without light perception.
After discharge, the patient was followed up at the hospital’s Ophthalmology Outpatient Clinic, where check-ups were scheduled for the following six months. Further antimicrobial and anti-inflammatory treatment was prescribed, including topical fluoroquinolones, loteprednol, dexamethasone, and bromfenac. Ultimately, the patient achieved a best-corrected visual acuity (BCVA) of 0.5 in the right eye. Currently (at the four-year follow-up), the patient’s visual acuity in the right eye remains stable. Post-inflammatory floaters have largely resorbed, though a small nuclear and posterior subcapsular cataract has developed. The left eye remains without light perception.
CASE REPORT 2
The patient, an 81-year-old woman, presented to the Ophthalmology Department complaining of redness, pain, and blindness in the left eye, persisting for five days. Ophthalmic symptoms were accompanied by disturbances in consciousness and awareness, significant weakness, and fever reaching 41°C with large daily fluctuations. The patient had a history of heart failure, hypertension, and hypothyroidism.
On admission, the patient’s Vos was light perception with only temporal projection, and the intraocular pressure (Tos) was 60 mmHg.
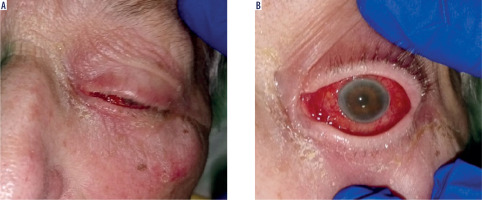
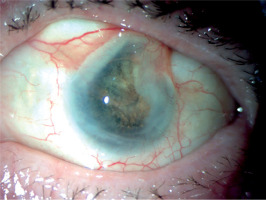
Physical examination revealed swelling and redness of the eyelids, deep mixed injection, corneal haze, fibrin in the anterior chamber with a plastron in the pupil lumen, hypopyon approximately 2 mm in size, and posterior iris synechiae (Figure 2). No fundus view was possible. B-scan ultrasound revealed numerous hyperechoic structures in the vitreous. The eye was severely tender to palpation.
The right eye exhibited no abnormalities and no signs of inflammation. The patient reported no history of trauma. A computed tomography (CT) scan showed no foreign body in the eye or orbit.
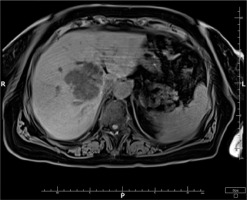
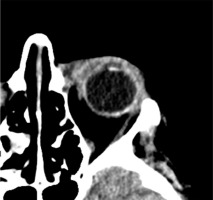
Based on the patient’s clinical presentation and history, a suspicion of endogenous endophthalmitis of the left eye was raised. An appropriate diagnostic work-up was performed, and empirical topical treatment (levofloxacin, dexamethasone, bromfenac, tropicamide, neosynephrine, brimonidine + timolol) as well as systemic treatment (ciprofloxacin, metronidazole, ceftriaxone) were initiated. Laboratory tests revealed elevated levels of inflammatory markers: CRP 226.1 mg/l, ESR 89 mm/h, procalcitonin 24.11 ng/ml, and leukocytosis 24.7 thousand/ml. Additionally, signs of renal impairment were observed with creatinine 148.1 μmol/l, urea 13.1 mmol/l, and eGFR at 31.22 ml/min/1.73 m2. Liver function tests showed slightly elevated ALT at 72 U/l and AST at 45 U/l. Furthermore, there was an increased D-dimer level of 1.828 ng/ml. Abdominal ultrasound revealed a hypoechoic structure in the liver, suggesting a suspicion of liver abscess. On CT scan, the lesion was identified as a well-defined structure measuring 61 × 60 × 46 mm, located at the perihilar region, on the border of segments IV, VIII, and VII. Magnetic resonance imaging (MRI) further revealed fluid signal intensity within the lesion and confirmed the presence of a thick-walled capsule (Figure 3). CT of the head showed increased density of the preseptal tissues in the left orbit (Figure 4).
The patient was transferred to the Department of General Surgery, where a laparotomy was performed. Using an ultrasound transducer, the abscess was located, evacuated, and drained. Intraoperatively, specimens were collected for culture and Klebsiella pneumoniae bacteria were isolated. No aerobic or anaerobic bacterial growth was detected in the blood cultures.
Systemic antimicrobial treatment was continued, and imipenem was added to the regimen. Topical therapy was maintained. Additionally, an intravitreal injection of an antibiotic (ceftazidime) at a dose of 2.25 mg/0.1 ml was administered. The patient did not consent to further injections.
Following stabilization of her general and local condition, the patient was discharged from the department after 10 days of hospitalization.
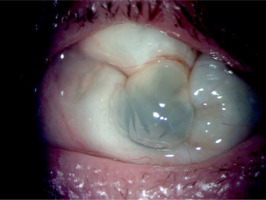
The treatment was continued at the hospital’s Ophthalmology Outpatient Clinic. Visual acuity in the left eye shows no perception of light. The eye became atrophied against the background of hypotonia, without accompanying pain (Figures 5 and 6).
DISCUSSION
Unlike exogenous endophthalmitis, the pathogenesis of endogenous endophthalmitis is not well understood. Knowledge regarding the diagnosis, management, and treatment outcomes relies primarily on isolated case reports [7-16]. No data coming from large multicenter studies or clinical observations are available.
There are several aspects differentiating endogenous from exogenous endophthalmitis. A medical history of recent ocular trauma or surgery typically prompts patients to seek immediate ophthalmic attention. Such patients are quickly and accurately diagnosed, and targeted therapy is implemented early. Patients with endogenous endophthalmitis often seek help from physicians of other specialties because of systemic symptoms. Often, the patient is unconscious, so no subjective ophthalmic symptoms are reported. It then becomes the responsibility of the attending physician – usually an anesthetist, internist, or surgeon – to suspect that the eye may be affected by a pathological process.
This disease is rare, affecting less than 0.04% of patients with general bacteremia [5]. However, in patients with systemic infection caused by Klebsiella pneumoniae, its prevalence ranges from 3.8% to 11% [8].
The most common underlying diseases leading to endogenous endophthalmitis include liver abscess (19%), respiratory infection (8%), and infective endocarditis (8%) [17]. Factors predisposing to the occurrence of the condition encompass recent hospitalizations, immunocompromised states, chronic diseases (such as diabetes, kidney failure, and cancer), invasive medical procedures, intravenous drug abuse, pregnancy and childbirth, and dental procedures.
Endophthalmitis can sometimes be the initial manifestation of a latent inflammatory focus [18-22], as in the cases discussed.
Diagnosis is challenging due to the low prevalence of the condition, diverse clinical manifestations, and similarities to other ophthalmic disorders. It may present with varying degrees of reduced visual acuity, eye pain, photophobia, swelling and redness of the eyelids, conjunctival injection, perilimbal hyperemia, corneal edema, and anterior chamber effusion or hypopyon. Depending on the severity of inflammation in the vitreous, various signs may be observed in the fundus, including flame-shaped intraretinal petechial hemorrhages, Roth spots, cotton wool spots, necrotizing retinitis, or simply inflammatory floaters or red reflex.
The condition is often (in 16-63% of cases) misdiagnosed [4]. The differential diagnosis primarily includes uveitis, keratitis, acute glaucoma attack, cancer dissemination, and conjunctivitis, especially in the early stages of progression.
Suspected endogenous endophthalmitis requires prompt and meticulous investigation into the underlying cause, involving a multidisciplinary team of specialists. The diagnostic work-up is complex and involves collecting samples of blood and other body fluids for culture, including urine, cerebrospinal fluid, synovial fluid, and abscess contents, depending on the clinical situation.
Diagnostic imaging (including scans of the head, chest, and abdomen) is another valuable modality for identifying the origin of infection. In the presented cases, CT of the abdominal cavity facilitated diagnosis and guided further treatment decisions.
Patients with endogenous endophthalmitis are typically in a serious general condition. Early initiation of treatment is linked to improved prognosis, both in terms of patient survival and visual function outcomes.
The priority is to promptly initiate empirical intravenous treatment with broad-spectrum antibiotics, including vancomycin, aminoglycosides, and third-generation cephalosporins. Systemic therapy also prevents the pathological process from involving the healthy eye.
Empirical intravitreal treatment involves administering vancomycin at a dose of 1 mg/0.1 ml along with either ceftazidime at 2.25 mg/0.1 ml or amikacin at 0.4 mg/0.1 ml. After obtaining the results of cultures and antibiograms, adjustments to this treatment may be made as necessary. Intravitreal drug administration ensures rapid and efficient delivery directly to the infection site to achieve high local levels where the causative pathogen is concentrated. This approach leads to improved treatment efficacy. Vitrectomy represents an alternative therapeutic option for topical ophthalmic treatment. The procedure is recommended for severe, vision-threatening endophthalmitis or when there is no response to systemic and topical therapy. It serves both therapeutic and diagnostic purposes [23, 24]. Vitrectomy was not performed in the presented cases. In the first case discussed, the procedure was not possible due to the patient’s severe general condition and treatment in the Intensive Care Unit. In the second case, the patient did not consent to vitrectomy.
The outcomes of treatment with regard to visual function are typically unsatisfactory. Vision loss in patients with endogenous endophthalmitis, resulting in the absence of light perception, evisceration, or enucleation, occurs in 55-69% of cases [25]. The final visual acuity is light perception or worse in 89% of patients [26].
Early diagnosis is crucial. On average, symptoms persist for one week before diagnosis. However, approximately 25% of patients experience symptoms for one to two weeks before the condition is diagnosed [27, 28]. A delay of more than two days in initiating appropriate treatment is associated with a poor prognosis for final visual acuity, as reported by Chou et al. [29]. In Case 1 discussed above, early initiation of comprehensive topical and systemic treatment resulted in a favorable outcome, with functional visual acuity preserved in one eye. Case 2 illustrates the consequences of delayed initiation of therapy due to the patient’s late presentation to the Department. Another factor contributing to worse prognosis was the patient’s refusal to consent to continue treatment with intravitreal injections.
Initial poor visual acuity is also known to correlate with a poorer prognosis for final visual acuity. Favorable outcomes can be anticipated in patients whose visual acuity is equal to counting fingers or better prior to initiating therapy [30]. In our study, patients initially presented with comparable baseline visual acuity (perception of light with full and partial projection), but the timing and scope of treatment administered resulted in significantly varied outcomes.
CONCLUSIONS
Endogenous endophthalmitis resulting from a liver abscess is a rare yet significant clinical issue, characterized by rapid progression and an unfavorable prognosis. In the presented cases, early diagnosis and prompt implementation of broad-spectrum antibiotic therapy, along with surgical treatment, led to patient survival. Moreover, in one of the reported cases, early initiation of topical treatment and intravitreal antibiotic injections enabled the patient to achieve functional visual acuity ensuring future independence in performing activities of daily living.
Rapid, aggressive, multidisciplinary therapy is essential to achieve optimal treatment outcomes and prevent complications.
It is recommended that patients with known or suspected Klebsiella pneumoniae liver abscess undergo regular ophthalmic check-ups, even in the absence of ocular symptoms at a specific time point.
It is crucial to educate and raise awareness among physicians from various specialties, including surgeons, internists, and anesthetists, about the clinical challenge posed by endo- genous endophthalmitis.

 ENGLISH
ENGLISH